冻干袋
lyoprotect冻干袋cGMP

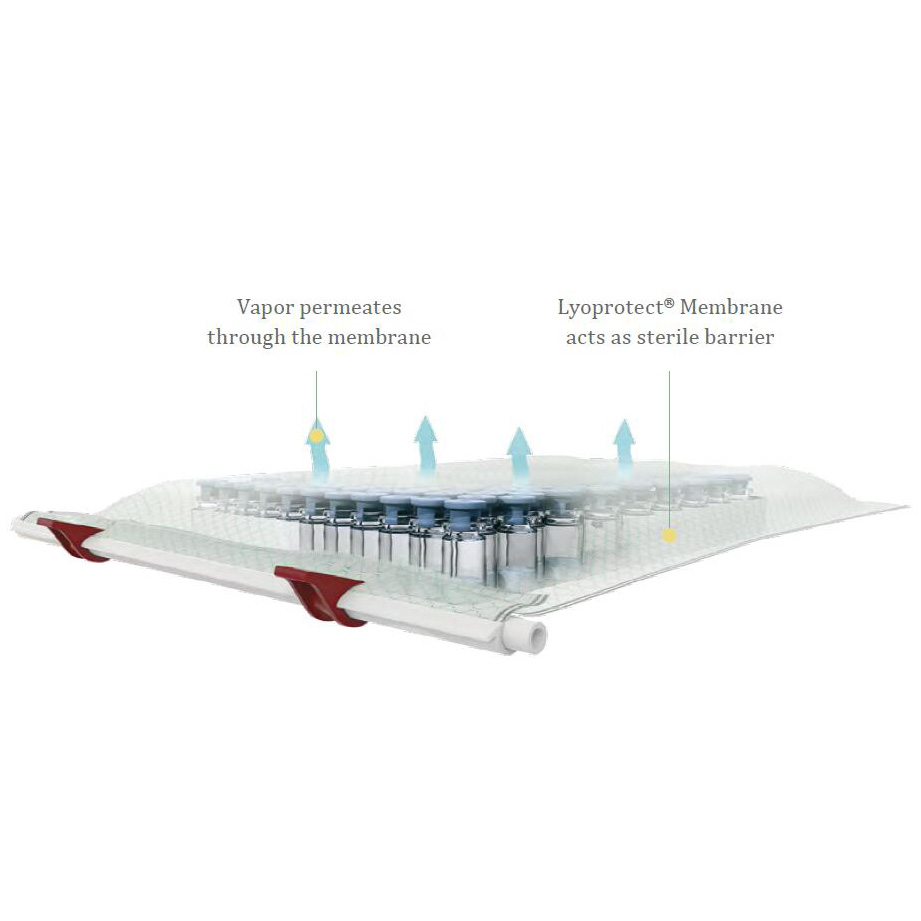
If aseptic criteria must be met, filling of vials, placement and closure of the bag
needs to take place under a laminar flow/working bench. And all required acces-sories (vials, stoppers, bag, closing stick etc.) need to be sterile.
But as soon as the bag is closed, you can move your assembly to any freeze-drier
in your facility. Your drug product will be safe - the Lyoprotect@Membrane acts as
a sterile barrier.

导航栏目
联系我们
斯沃德.中国电话:+86-010-62894911
传真:+86-010-62894911.
手机:13910235149
地址:北京市海淀区天秀路10号中
国农业大学国际创业园5018室
邮箱:de-source@163.com
网址:www.seaworld1.com
友情链接:
www.bionet.com
www.fiocchetti.it
www.coolvacuum.es
www.www.myr.com.es